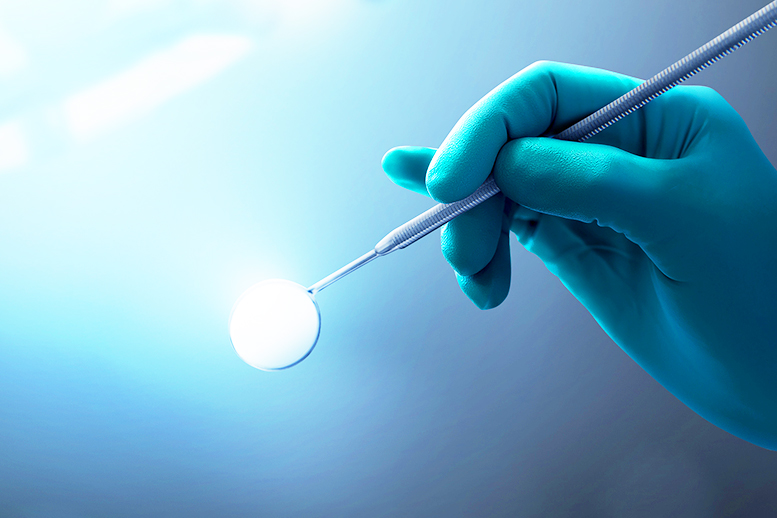

Efficient Production:The workshop adheres to the principles of "process optimization, safety, and compliance" to achieve seamless production.
Management System:A comprehensive personnel management, production process management, and quality control system are in place.
Green Production:Energy-saving and emission-reduction measures are implemented throughout all production processes to reduce waste and help the factory achieve synergistic economic and environmental benefits.
Click to Explore More

PRODUCT
Ascent Petrochem Holdings Co., Limited

Acrylic Acid

Methyl Acrylate

Ethyl Acrylate

Butyl Acrylate